About Rafias
Building the Future of Addiction Treatment
The Challenge
Addiction affects millions worldwide, and relapse during abstinence remains the hardest problem to solve. Stimulant use disorders have no FDA-approved medications, and behavioral therapies alone lead to high relapse rates and costly inpatient care.
Rafias is pioneering the first pharmacologic therapy engineered to prevent relapse during the abstinence phase, when the stakes are highest and current treatments fail.
Our Mission
We are developing the first safe and effective medication to prevent relapse in stimulant use disorder. Our work advances neurobiology and chemistry to restore the brain’s capacity for sustained recovery.
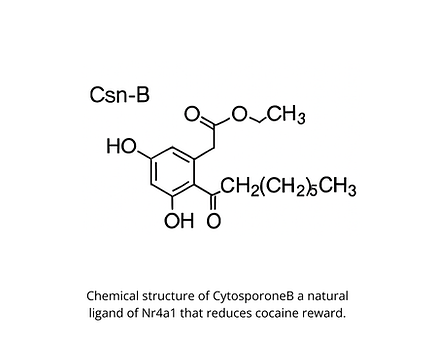
Rafias targets Nr4a1, a transcription factor linked to addiction resilience. Through translational research at the University of Pennsylvania, we’re validating Nr4a1 as a first-in-class therapeutic target and building a commercially viable drug development pipeline.
The Science

Nr4a1 regulates expression of downstream effector genes through chromatin remodeling. These effector genes restore brain signaling to reduce the rewarding effects of cocaine. Yet this function is delayed until late in abstinence. Our novel compound activates Nr4a1 in early abstinence to promote recovery before relapse can occur.
NIH-Funded Innovation, Backed by Penn Research

$405K
NIH STTR Phase 1 Grant (Lead Identification)
$230K
NIH U18 Grant (Target Discovery)
$1M
NIH STTR Phase II (Prototype Development, pending)
Our Focus Areas
Advancing science toward the first treatment for cocaine use disorder.
Target Discovery
Rafias discovered that Nr4a1, a transcription factor, is linked to addiction resilience. Activating Nr4a1 reduces drug-seeking behavior and restores neural balance in preclinical models.

Drug Development
We are designing next-generation Nr4a1 activators with improved stability and specificity. Each candidate undergoes rigorous potency, selectivity, and safety screening.
Translational Research
Preclinical studies confirm efficacy disorder and safety benchmarks, bridging foundational neurobiology with therapeutic application.

Platform & Partnerships
Supported by NIH Small Business Funding and Penn's Center for Innovation, Rafias is building a first-in-class platform to deliver safe, effective addiction therapeutics to market.
.png)





-processed_edited.jpg)
%20(1000%20x%20938%20px)%20(12)_edited.jpg)

