For Investors

Overview
Addiction affects millions worldwide, and relapse during abstinence remains the hardest problem to solve.
Despite the scale of the crisis, stimulant addiction has no FDA-approved medications—leaving a multibillion-dollar gap in effective treatment options.
Rafias is building the first targeted therapeutic designed specifically to prevent relapse by restoring adaptive neural function.


The Market Need
-
Addiction is a chronic, relapsing disorder
-
Stimulant addiction is rising globally
-
No pharmacologic treatments currently exist
-
Behavioral therapies lead to high relapse rates (40–60%)

A Global Crisis Demanding Scalable, Science-Driven Solutions
By The Numbers
Why Now?
Structural shifts in addiction treatment, neuroscience, and funding make this the right moment for targeted relapse-prevention therapeutics.
No FDA-Approved Treatments
Behavioral therapy remains the only option for stimulant addiction, despite relapse rates exceeding 40–60%. A pharmacologic solution addresses a massive, unmet clinical need.
NIH-Funded Validation
Multiple NIH awards, including U18 and STTR grants, support the feasibility of Rafias’ platform. Non-dilutive funding significantly de-risks early R&D.
Growing Market Pressure
Stimulant addiction is rising globally, with no existing pharmacologic interventions. Providers, health systems, and payers urgently need relapse-prevention options that reduce long-term costs.
Strong Scientific Foundation
Over a decade of neuroscience and epigenetics research supports Nr4a1 as a first-in-class therapeutic target. Peer-reviewed studies demonstrate its role in reducing drug-seeking behavior and restoring neural stability.
Clear Regulatory Pathway
Addiction therapeutics with strong biological rationale have favorable pathways for IND-enabling work and early-phase trials. Regulatory agencies are increasingly prioritizing new addiction treatments.
First-Mover Advantage
Rafias is positioned to establish the first targeted, abstinence-phase therapeutic—creating a new category in addiction treatment with significant commercial and clinical potential.
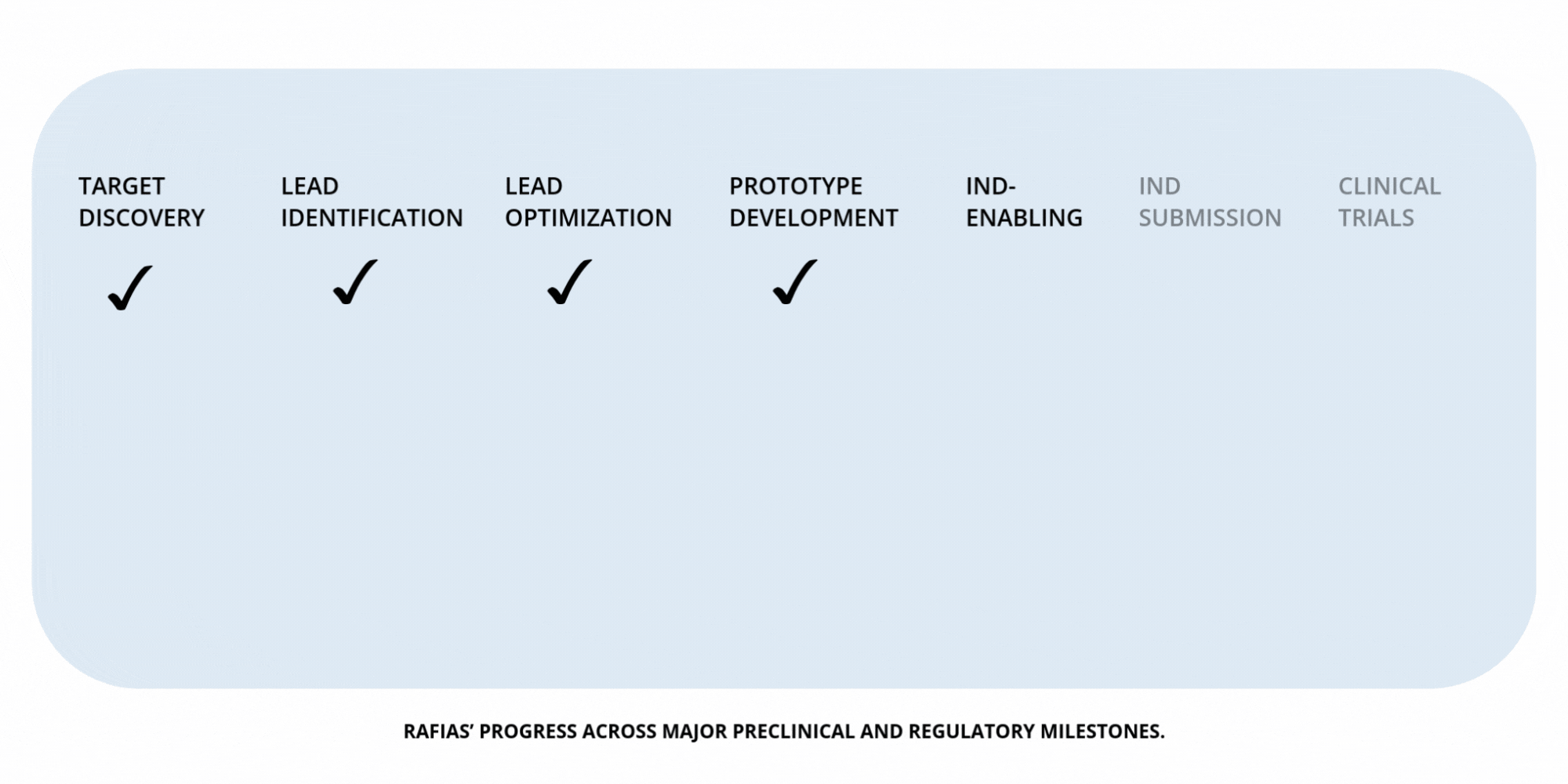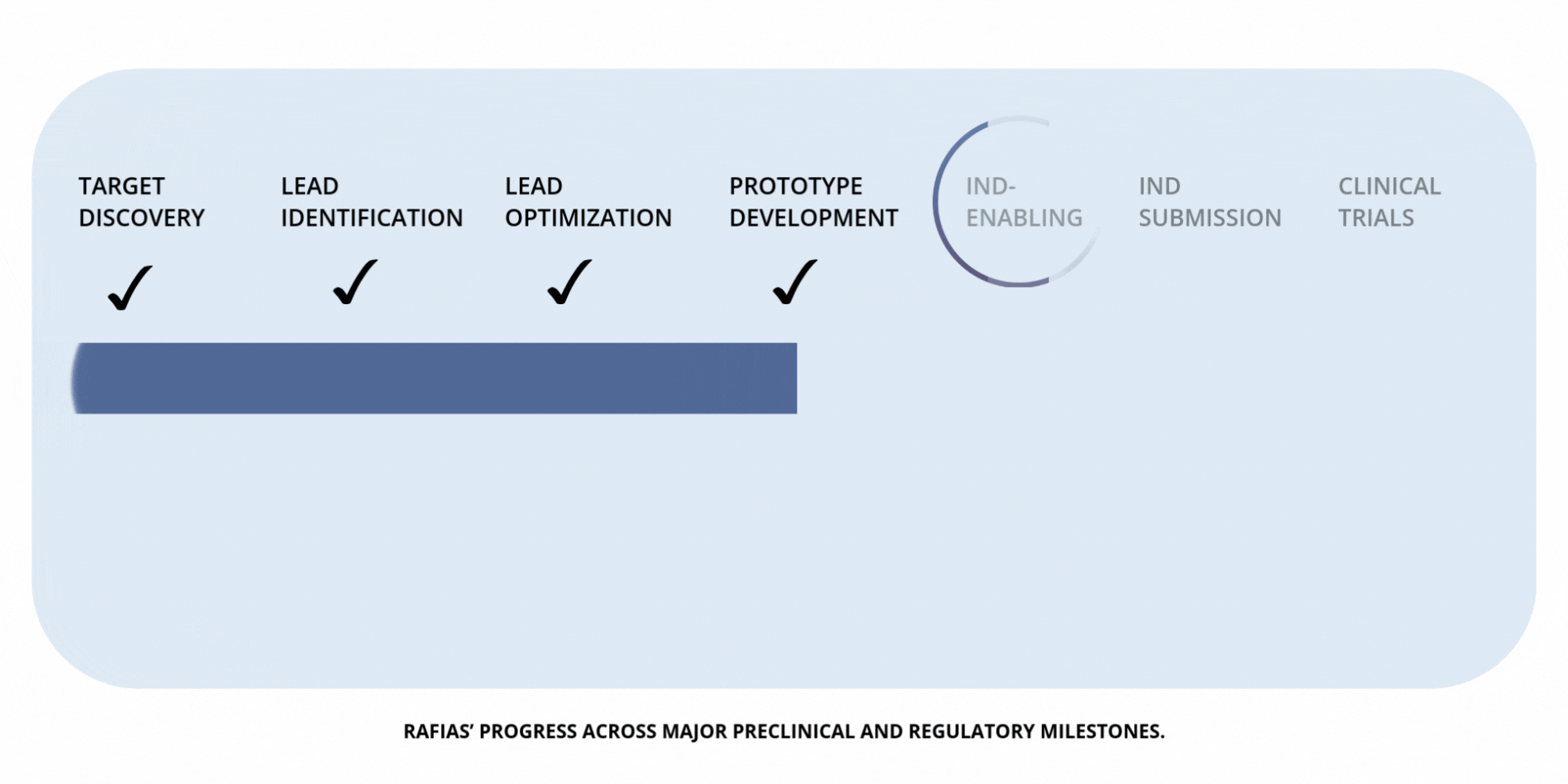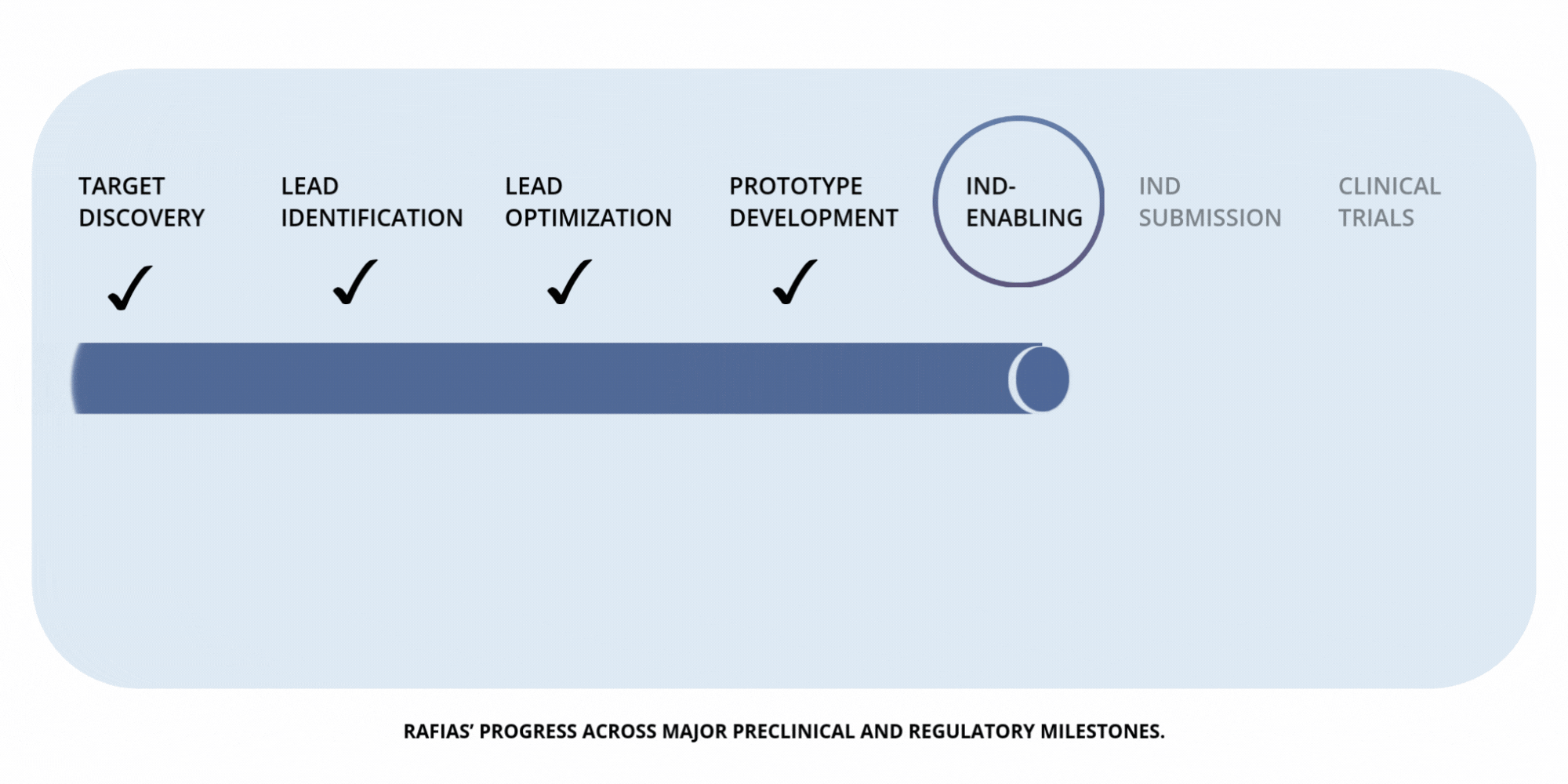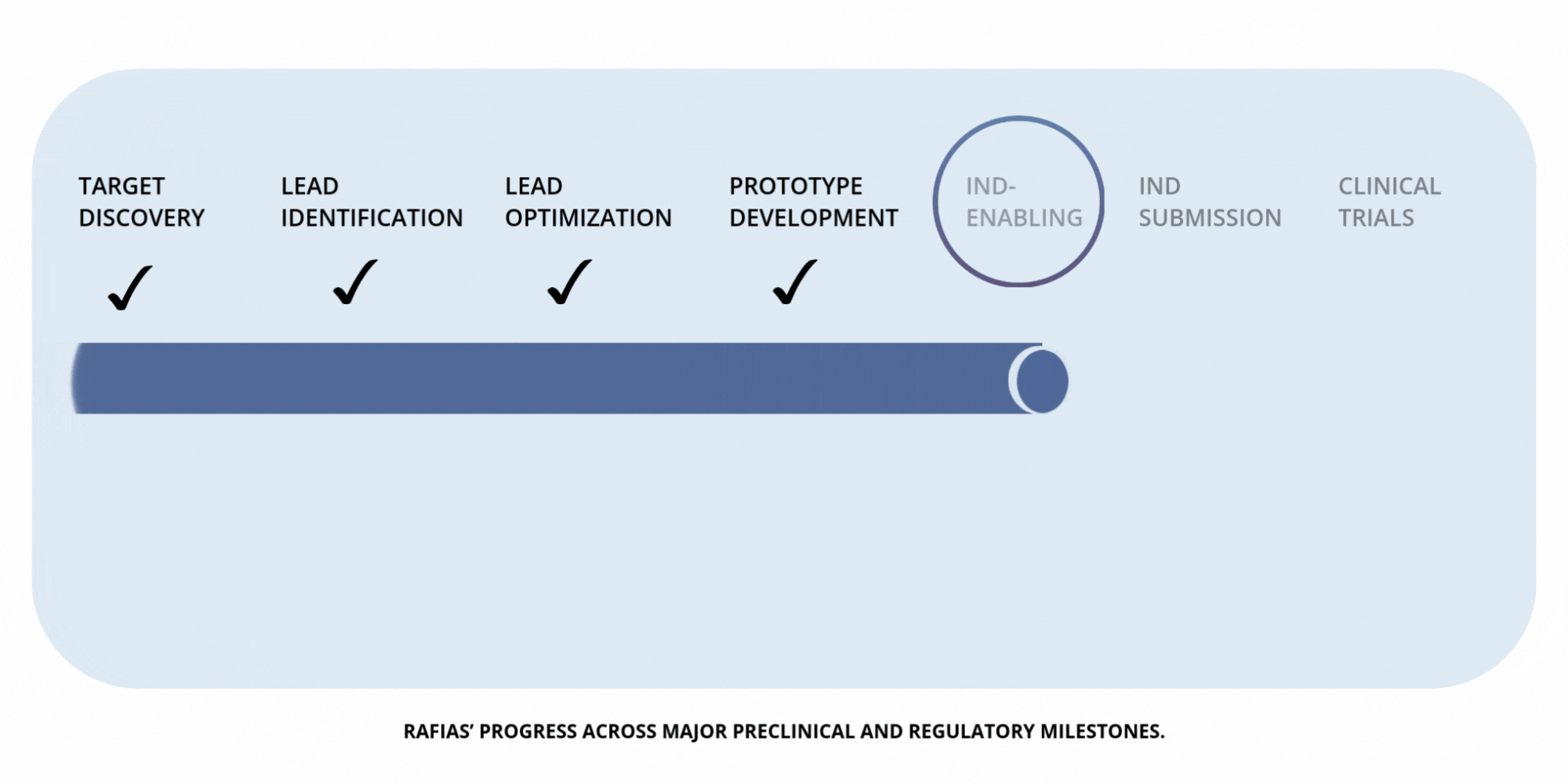
Development
Timeline

NIH U18 Grant — Target Discovery
Supports mechanistic validation of Nr4a1 as a therapeutic target in addiction, establishing its role in neural homeostasis and relapse biology.
NIH STTR Phase I — Lead Identification
Funds the design and early evaluation of Nr4a1-directed molecules, identifying promising candidates for further optimization.
NIH STTR Phase II — Prototype Development (pending)
Supports optimization and preclinical testing of therapeutic prototypes aimed at restoring recovery pathways during abstinence.
Preclinical Milestones — IND-Enabling Work (in progress)
Advancing pharmacology, safety, and proof-of-concept studies required to prepare for IND submission.
IND Submission (planned)
Regulatory milestone preceding human trials.
Clinical Trials — Phases I–III (future milestone)
Evaluation of safety, dosing, efficacy, and long-term relapse-prevention outcomes.

Funding & Grants
Rafias’ platform is supported by multiple NIH awards, including a U18 Target Discovery grant and Phase I STTR funding for lead identification. Additional STTR Phase II funding is pending. These awards validate scientific feasibility and provide non-dilutive support for the next stages of development.
Looking Ahead
Rafias’ platform is supported by multiple NIH awards, including a U18 Target Discovery grant and Phase I STTR funding for lead identification. Additional STTR Phase II funding is pending. These awards validate scientific feasibility and provide non-dilutive support for the next stages of development.